Hepatitis C virus
A suspicion that other viruses were involved in post-transfusion-(PTF) related hepatitis was first aired by Harvey J Alter and colleagues, after proof in 1972 that some PTF-related hepatitis cases had no HBV antigen associated with them. By 1977 hepatitis A virus (HAV, infectious hepatitis) had been excluded as well, and the term “non-A, non-B” hepatitis (NANB) was coined. By 1978, transmission studies using human serum injected into chimpanzees showed that
“Hepatitis was transmitted by serum derived from patients with chronic as well as acute hepatitis, strongly suggesting a chronic carrier state for the agent responsible for non-A, non-B hepatitis. Non-A, non-B hepatitis thus seems to be due to a transmissible agent which can persist and remain infectious for long periods”.
There was also evidence from Japan the same year that there might be a novel antigen – hepatitis C (HC) antigen – associated with NANB PTF hepatitis. In 1979, it was suggested from ultrastructural studies in cells from infected chimpanzees that more than one NANB agent might exist; by 1980 Alter had concluded that that the NANB hepatitis agent(s) played a dominant role in the pathogenesis of PTF hepatitis. In 1987, in an interesting application of essentially the same technology used to characterise the first viruses, Alter’s group used polycarbonate membranes to filter the infectious agent, and showed it was 30-60 nm in diameter, and therefore highly unlikely to be a retrovirus, as had been suggested by some.
Also in 1987, Michael Houghton, Qui-Lim Choo, and George Kuo at Chiron Corporation collaborated with DW Bradley at the CDC in using the much newer technology of constructing a random-primed cDNA clonal library from RNA extracted from human plasma in a lambda phage expression vector, and screening proteins expressed from the library against NANB hepatitis-infected patient serum. They discovered one sequence that produced a protein fragment that bound antibodies, sequenced it, and used the sequence to “primer walk” through the entire genome by repeated cDNA generation, cloning and sequencing. They published their finding in 1989 of
“…an RNA molecule present in NANBH infections that consists of at least 10,000 nucleotides and that is positive-stranded with respect to the encoded NANBH antigen. These data indicate that this clone is derived from the genome of the NANBH agent and are consistent with the agent being similar to the togaviridae or flaviviridae”.
The agent was unique among viruses characterised until then, as no virus particle had yet been seen, let alone isolated. Alter and his team meanwhile tested for the presence of the virus in NANB patient samples, and in 1989 also published a paper – back-to-back with the previous – on
“An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis”.
The sequence of the putative agent allowed cloning and expression of a putative capsid protein in yeast, which allowed large-scale screening of patient samples and donated blood. From their paper:
“These data indicate that HCV is a major cause of NANBH throughout the world”.
There was also already evidence that NANBH was associated with hepatocellular carcinoma (HCC) in Japan: M Sakamoto and colleagues showed that 90% of HBsAg-seronegative patients, who were also overwhelmingly HBV DNA-negative, showed evidence of chronic hepatitis in the non-cancerous liver, and 29% had a history of blood transfusion. This was followed as early as 1989 by evidence that 65% Italian HCC patients had antibodies to HCV, and again the same year by evidence from Spain that 75% of HCC patients had HCV antibodies, which
“…indicate[s] that HCV infection may have a role in the pathogenesis of hepatocellular carcinoma, even in patients with chronic liver disease apparently related to other agents such as alcohol”.
By 1990, K Kiyosawa and colleagues felt able to state that:
“These data suggest the slow, sequential progression from acute hepatitis C virus-related non-A, non-B hepatitis through chronic hepatitis and cirrhosis to hepatocellular carcinoma and support a causal association between hepatitis C virus and hepatocellular carcinoma”.
These findings rapidly led to the revelations that hepatitis C virus (HCV) was implicated in both an acute and relatively mild illness that lasts only a few weeks, and a chronic form that is usually more serious and can last lifelong. Between 15–45% of infected persons spontaneously clear the virus within 6 months; however, the remaining 55–85% will develop chronic HCV infection.
Up to 150 million people globally are chronically infected with HCV. Moreover – also from the WHO site – a significant number of these people will develop liver cirrhosis or liver cancer, and up to 500 000 people die worldwide every year from HCV-related liver disease.
There is as yet no vaccine against HCV infection, although trials are quite far advanced – and one candidate combination prime-boost strategy from Eleanor Barnes and coworkers seemed to show promise as of the end of 2014. This consisted of
“…a replicative defective simian adenoviral vector (ChAd3) and modified vaccinia Ankara (MVA) vector encoding the NS3, NS4, NS5A, and NS5B proteins of HCV genotype 1b” – which is using two well-characterised viruses as gene vectors to combat a third.
The authors make the point that the responses they achieved in human volunteers are similar to those seen in people who control natural infections.
Meanwhile, chemotherapy for chronic infections is both a realistic and well-established area: there are a number of treatments on the market already, and there have been significant recent developments which may make treatment even more effective.
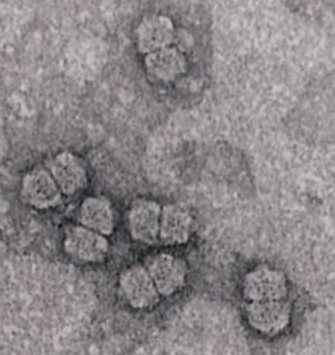
HCV particles were finally characterised from cell culture-grown virus: both enveloped and non-enveloped pleomorphic spherical particles were found, of around 60 nm and 45 nm in diameter respectively. This agrees well with the estimation by filtration of 30-60 nm previously determined in 1987. The virus is classified as a ss(+)RNA genome flavivirus, similar to yellow fever virus, in the genus Hepacivirus, family Flaviviridae
Kaposi sarcoma herpesvirus
Moritz Kaposi in 1872 described what was originally called an “idiopathic multiple pigmented sarcoma of the skin”, which present as
“…disseminated blood- or bruise-coloured skin lesions (flat plaques or nodules) in the skin, usually on the lower extremities though sometimes on the hands and arms”.
What was subsequently called Kaposi Sarcoma, or KS, was at first thought to occur only among elderly men of Jewish, Arabic or Mediterranean origin; however by the 1950s it was realised it was quite common in sub-Saharan Africa, which led to the first suggestions that it might be caused by a virus.
In 1981-1982, however, the CDC received reports of KS occurring in otherwise healthy young homosexual men in California – often together with Pneumocystis carinii pneumonia, which was also previously very rare. The disease was also much more aggressive, and spread beyond the skin into other tissues including bone, the mouth, gastrointestinal tract and lungs.
While there was at this time still no clue as to why this should be, the link to sexual acts as well as the previous observation that KS occasionally appeared in immune-suppressed organ transplant patients, led epidemiologists to discover the sexual transmission of immunodeficiency that led to the discovery of HIV and its causation of AIDS.
Valerie Beral and colleagues working at the CDC in the late 1980s used epidemiological data on KS in AIDS patients to build a compelling case for the tumour being caused by another sexually transmitted virus. In a landmark paper in The Lancet, they announced in 1990, on the basis of painstaking and traditional-style investigation of 8 years’ worth of information from more than 90 000 people with AIDS collected in the US by the CDC since 1981, that:
“In the United States Kaposi’s sarcoma is at least 20000 times more common in persons with [AIDS] than in the general population and 300 times more common than in other immunosuppressed groups…Kaposi’s sarcoma was commoner among those who had acquired the human immunodeficiency virus (HIV) by sexual contact than parenterally, the percentage with Kaposi’s sarcoma ranging from 1% in men with haemophilia to 21% in homosexual or bisexual men. Women were more likely to have Kaposi’s sarcoma if their partners were bisexual men rather than intravenous drug users”.
The UK Cancer Research site on KS has an excellent account of the study as well as of its impact – one aspect of which was the proof in 1994 that indeed a virus was involved, using modern. This was published by Y Chang and colleagues in Science, and detailed the use of the very modern technique of:
“Representational difference analysis … to isolate unique sequences present in more than 90 percent of Kaposi’s sarcoma (KS) tissues obtained from patients with acquired immunodeficiency syndrome (AIDS). These sequences were not present in tissue DNA from non-AIDS patients, but were present in 15 percent of non-KS tissue DNA samples from AIDS patients. The sequences are homologous to, but distinct from, capsid and tegument protein genes of the Gammaherpesvirinae, herpesvirus saimiri and Epstein-Barr virus. These KS-associated herpesvirus-like (KSHV) sequences appear to define a new human herpesvirus.”
This became human herpesvirus 8 (HHV-8), the newest of the seven viruses known to cause human cancers.
Adeno-associated virus
A report in Nature Genetics in August 2015 implicates adeno-associated virus type 2 (AAV2) in causation of human hepatocellular carcinoma (HCC) – specifically by means of insertional mutagenesis, in “cancer driver” genes. Clonal integrations – with the same genome at the same site(s) – were found in 11 of 193 HCCs sampled, and the authors noted multiple insertions in some tumours, with significant effects on gene expression.
It is especially interesting that tumours with integrated viral genomes were found in non-cirrhotic liver (9 of 11 cases) and tumours in patients without known risk factors (6 of 11 cases). The authors suggest a clear pathogenic role for AAV2 in these cases, and conclude that “AAV2 is a DNA virus associated with oncogenic insertional mutagenesis in human HCC”.