PBVAB 5 Verona, June 2013 – Part 3
Technically, Sue Huddy’s piece should have been Part 3; however, it reports things that happened after what I am reporting on, so I’ll keep that label!
This post will report on Sessions 3 & 4, namely, Technology Advances and Perspectives.
I opened Session 3 with a talk on ‘Virus-derived ssDNA vectors for the expression of foreign proteins in plants’, focusing mainly on geminiviruses (naturally). I wrote this a couple of years ago as a chapter for a book which seemed to not be forthcoming; however, I was assured during my talk by Yuri Gleba – the co-Editor with Kenneth Palmer of a “Current Topics in Microbiology and Immunology” issue on “Plant Viral Vectors” – that this offering is now in fact available, so here’s a link for anyone who wants to buy it.
Current Topics in Microbiology and Immunology 2011,
Virus-Derived ssDNA Vectors for the Expression of Foreign Proteins in Plants
Edward P. Rybicki, Darrin P. Martin
Plant viruses with ssRNA genomes provide a unique opportunity for generating expression vehicles for biopharming in plants, as constructs containing only the replication origin, with the replication-associated protein (Rep) gene provided in cis or in trans, can be replicationally amplified in vivo by several orders of magnitude, with significant accompanying increases in transcription and expression of gene(s) of interest. Appropriate replicating vectors or replicons may be derived from several different generic geminiviruses (family Geminiviridae) or nanoviruses (family Nanoviridae), for potential expression of a wide range of single or even multiple products in a wide range of plant families. The use of vacuum or other infiltration of whole plants by Agrobacterium tumefaciens suspensions has allowed the development of a set of expression vectors that rival the deconstructed RNA virus vectors in their yield and application, with some potential advantages over the latter that still need to be explored. Several modern applications of ssDNA plant vectors and their future potential will be discussed.
I noted that several firms are already using geminivirus-derived expression technology – like Kentucky Bioprocessing, who offer use of it as a service, and Medicago Inc, who use it in manufacturing vaccine products – and that it has considerable potential for improvement. There is also the possibility of using other ssDNA virus-derived vectors, including from bacteria.
E.V. Sheshukova (N.I. Vavilov Institute of General Genetics RAS, Moscow) followed up with an account of how the use of antisense RNA to plant death factor (PDF) could modulate PDF level so as to avoid the necrotisation caused by rapid protein over-expression. Their group used a TMV-based vector to co-express an antisense with the gene of interest, and got 4-5-fold increase in protein expression, equivalent to using the silencing suppressor p19 from a tombusvirus.
Diego Orzaez (IPMCP-CSIC, Valencia, Spain) spoke next, on the same technology I have previously described (with beautiful pictures from Diego) here: that is, the enabling of tools for multigene engineering of plants – and specifically in this case, the elegant use of superinfection exclusion phenomenon seen with RNA plant virus-derived vectors that are capable of movement, for the expression of polyclonal antibody mixtures in plant leaves. They had successfully shown expression of 300+ individual clones from a camel VHH clonal library derived against a mixture of 3 snake venoms, in a mosaic on a single leaf. This was seriously impressive for me: imagine, polyclonal “sera” from a leaf!
Diego noted that the FDA allows the 2-animal rule for products like antivenin, and things used for biodefence: that is, an efficacy trial in an animal, followed by Phase 1 trial in humans (=safety). This could help expedite approval of such products.
We discussed the paper previously blogged on from this group in Journal Club today, incidentally, to much appreciation of the truly excellent work, and the colour Figures. Thanks, Richard!
Reza Saberianfar (Agriculture and Agri-Food Canada, Ontario) described their investigations of protein body biogenesis in N benthamiana. They had looked mainly at hydrophobin and elastin fusion proteins, in order to overcome the joint bottlenecks of inadequate accumulation, and difficulties in purification of recombinant proteins from plants. He noted that hydrophobin and elastin PBs were different sizes: they had used protoplasts of infiltrated leaves and confococal microscopy and Imaris software to find every PB in individual cells, to determine that shows hydrophobin-based PBs were 1-2 um, and ELP-based were 2-3 um in diameter, for the same amount of protein. PBs made from hydrophobin and ELP-linked proteins shared the same ER origin, but Zera-based PBs had a different origin and Zera fusions did not need a KDEL for ER retention. An interesting observation was that PBs could form in the ER in the absence of fusion tags if expression levels were high. One could also increase the expression of other proteins by coexpressing them with a fusion protein, as they get incorporated into PBs anyway – eg: EPO.
Lauri Reuter (VTT-Technical Research Centre, Finland) continued in the theme of fusion proteins with a talk on the production of hydrophobin fusions in tobacco BY-2 suspension cultured cells. It was interesting to hear that WAVE bioreactors did not work well because they did not shake fast enough, but that conventional steel bioreactors did – with capacities of 20 – 600 litre, and even up to 20 m3. The cells are apparently surprisingly tolerant to shear stresses, and yields of GFP::hydrophobin fusion from 600 litre reactors were as good or better as from a 50 ml shake flask – at 300 mg/litre. Purification was simple, in that reactors could be pumped out onto a filter, and the cell “cake” pressed dry – for subsequent lyophilisation and storage at room temperature, for example. French pressing of fresh cells was also an option. Hydrophobin fusions allowed aqueous 2-phase separations, for simple and rapid enrichment. Inclusion of a Tobacco etch virus self-cleaving motif allowed removal of the hydrophobin.
 The “Perspectives” Session was notable for two talks, and a proposal: the latter was by Julian Ma for a “Society for Molecular Farming”, which was well supported and will probably kick off sometime this year.
The “Perspectives” Session was notable for two talks, and a proposal: the latter was by Julian Ma for a “Society for Molecular Farming”, which was well supported and will probably kick off sometime this year.
Jim Larrick (Panorama Research, Mountain View, California) gave a typically eclectic, wide-ranging and highly enthusiastic talk on ‘Anti-fragility: Big picture issues in pharmaceutical development’. He used the “Black Swan” analogy repeatedly to explain how the enterprise funding and pharma research sectors embodied fragile or anti-fragile thinking – with the observation that it was easier to resist black swans (eg: the unexpected) with a raft of small projects, than to have a few big ones. He also pointed out that the NIH liked big projects – and that a useful alternative name for them was “Not Invented Here”! Right up there with “Not Real Funding” as the alternative name for our National Research Foundation….
Matthew Paul (St. George’s University of London) presented a set of 15 case studies of commercial paths to introducing molecular farming, which was very interesting to us academic types. More interesting was the fact that while innovative and protectable technology and products were important to start-ups, the majority of successful ones had their basis in platform development – and the average time from platform to product identification was about five years. Venture capital firms were considered too greedy for early-stage start-ups, but their involvement later led to stability as their partnering was long term.
Another interesting feature was that many of the successful ventures sold “side products”: for example, Ventria sold cytokines and cosmetic formulations, while KBP sold cell culture reagents. Several also licenced out technology platforms, but the revenue was not held to be so good.
There were three main indicators of success:
- Management quality
- A good lead product
- Having a panel of products
 A good strategy to stay alive was “maximum income / minimum burn” – and he held up the example of Medicago in this regard. He noted that in the absence of major investment from Big Pharma, Phase 2 trial success was the driver for commercialisation.
A good strategy to stay alive was “maximum income / minimum burn” – and he held up the example of Medicago in this regard. He noted that in the absence of major investment from Big Pharma, Phase 2 trial success was the driver for commercialisation.

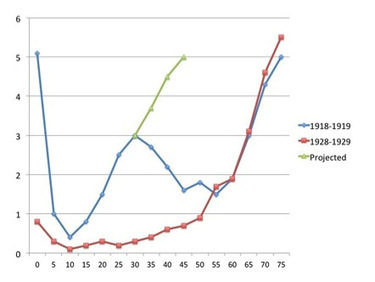





 have been thinking about this paper (see last post), and it and other people’s posts (eg:
have been thinking about this paper (see last post), and it and other people’s posts (eg: